ARTISS

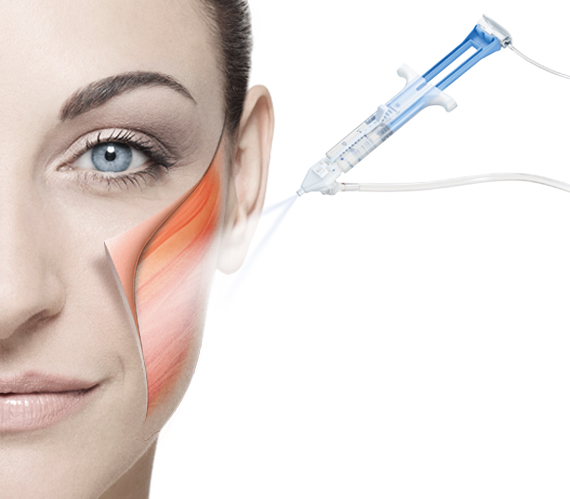
As the first and only fibrin sealant custom designed for tissue adherence in plastic, reconstructive and burn surgery, ARTISS is effective in a pivotal phase 3, multicenter, prospective, randomised, clinical study.1
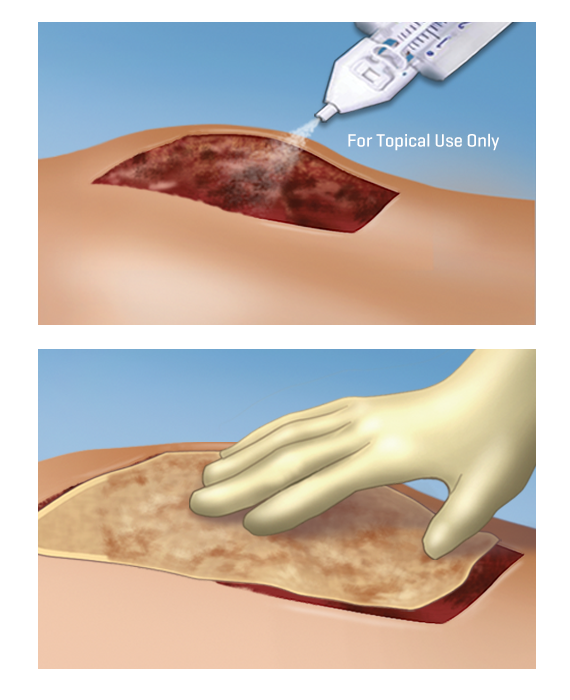
Mechanism of Action
ARTISS is a two component fibrin sealant, consisting of human thrombin and human fibrinogen and an antifibrinolytic inhibitor to delay clot degradation.2
Upon mixing, soluble fibrinogen is transformed into a fibrin matrix that adheres to the wound surface and to the skin graft to be affixed.2
Initial polymerisation of ARTISS will take up to 60 seconds due to optimised thrombin concentration; allowing time to manipulate and position the graft prior to polymerisation.2
The adhesive properties of ARTISS provide full surface adherence of the graft to the wound bed, closing the space that exists when grafts are attached using point fixation techniques such as staples.1
ARTISS may reduce or eliminate the need for staple application or removal.1
Additional Product Benefits
Significantly Reduced Drainage Volume
In facial rhytidectomy, drainage volume was reduced on average by 62%, when evaluated 24 hours (+/- 4h) post-surgery, when ARTISS was used with the standard of care (SoC) vs. SoC alone (7.7 ± 7.4 mL vs 20.0 ± 11.3 mL (p < 0.0001), n=75). ARTISS may also eliminate the need for surgical drains.3
Effective for Complete Wound Closure
In burn surgeries, ARTISS has been demonstrated to be better than staples, with 43.3% of sites achieving full wound closure by day 28 compared to 37.0% for staples.2
Flexible and Convenient
ARTISS allows up to 60 seconds to manipulate and position the tissue flap. In addition, it doesn’t require any mixing or reconstitution, and it may be stored for up to 14 days at controlled room temperature (not exceeding +25°C) when unopened pouches are thawed at room temperature.3
Prescribing Information:
| UK ARTISS |
Adverse Events and any drug or medical device product quality complaints (including suspected defective medicines or medical device adverse incidents) should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.
Adverse Event and Product Complaint Handling
Adverse Events should also be reported to Baxter Healthcare Ltd, by email ([email protected]) or by phone (+44) 01635 260360
Drug or medical device product quality complaints relating to Baxter products can be reported directly to Baxter Healthcare Ltd by email ([email protected]) or by phone (+44 1604 704603).