VIBe SCALE
By utilising the Validated Intraoperative Bleeding Scale (VIBe SCALE), surgeons could reduce intraoperative & post operative complications by choosing the right adjunctive topical haemostatic product for the right clinical situation.1
This site is intended for Healthcare Professionals only.
Important Safety InformationBaxter’s programs and partnerships provide a holistic approach to optimising patient care, reducing costs, and advancing the art of healing
Enabling surgeons to act with precision and speed to improve outcomes, minimize complications and increase efficiencies
Empowering surgeons to make the critical decisions that can impact longer, and healthier lives
The solutions surgeons rely on to promote healing
Helping surgeons solve the challenges their patients need them to solve
Support for product preparation for surgical teams
Baxter’s programs and partnerships provide a holistic approach to optimising patient care, reducing costs, and advancing the art of healing
The common language for patient blood management
Data-driven, evidence-based hemostasis optimisation program
A collaborative community of healthcare professionals
Surgeries in the 21st century continue to evolve with new complexities. Baxter's comprehensive portfolio of products provides evidence-based, cost-effective solutions.
This site is intended for Healthcare Professionals only.
Important Safety InformationBy utilising the Validated Intraoperative Bleeding Scale (VIBe SCALE), surgeons could reduce intraoperative & post operative complications by choosing the right adjunctive topical haemostatic product for the right clinical situation.1

Kidney Abrasion. Grade 0 reflects clinically insignificant and unremarkable bleeds.

Capsular Liver Abrasion. Grade 1 Bleeds represent a general ooze, which well up over 1-2 minutes after blotting with gauze.
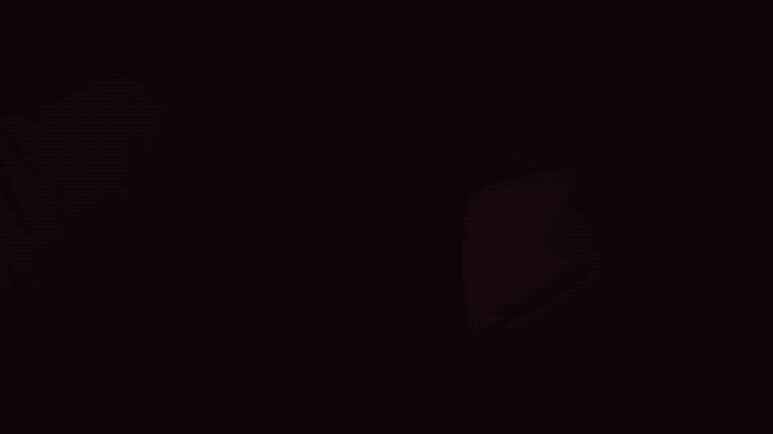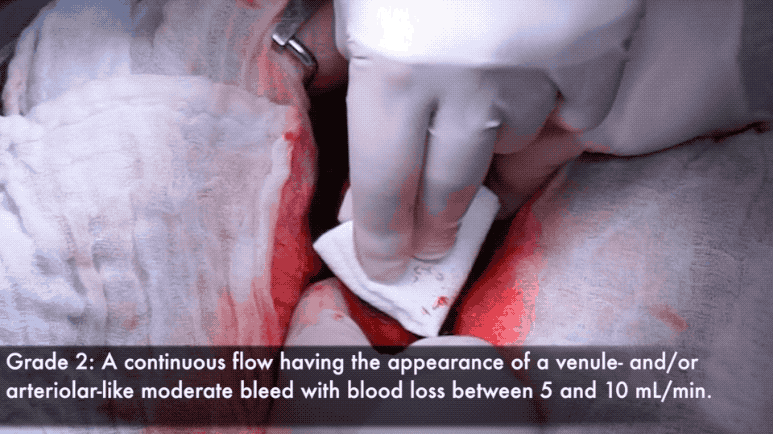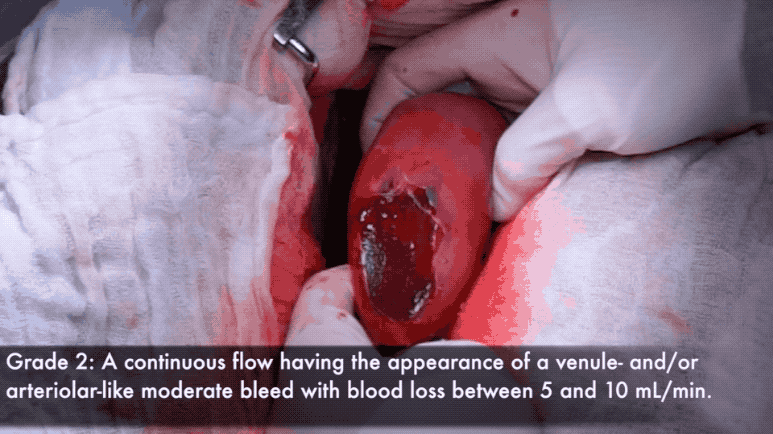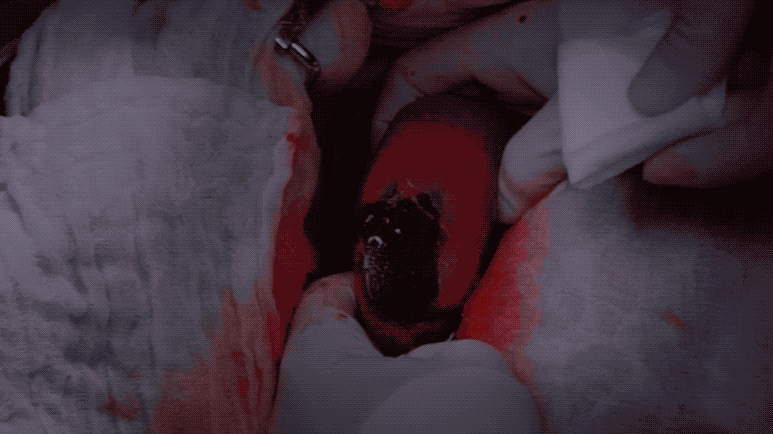
Grade 2 bleeds visibly well up after blotting, and are usually considered distracting to the surgical procedure.

Rupture of venous plexus during posterior lumbar laminectomy. Grade 3 bleeds well up immediately after blotting, and require treatment to continue with the surgery.

Abdominal Aortic Tear. Grade 4 bleeds are life-threatening and require immediate surgical treatment.
Prescribing Information:
| UK TISSEEL |
Adverse Events and any drug or medical device product quality complaints (including suspected defective medicines or medical device adverse incidents) should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.
Adverse Event and Product Complaint Handling
Adverse Events should also be reported to Baxter Healthcare Ltd, by email ([email protected]) or by phone (+44) 01635 260360
Drug or medical device product quality complaints relating to Baxter products can be reported directly to Baxter Healthcare Ltd by email ([email protected]) or by phone (+44 1604 704603).
Lewis KM, et al. Development and validation of an intraoperative bleeding severity sale for use in clinical studies of haemostatic agents. Surgery 2017;161:771-81.
Please click below to confirm you are a healthcare professional